Antibiotic resistance: towards drugs to disarm bacteria
An interdisciplinary research team has identified a molecule that is able to disarm pathogenic bacteria and thus support our immune system. This happens without any negative effects on the host's microbiota. The study was coordinated by INRAE, the researchers are part of the CMFI at the University of Tübingen, the CNRS, the University of Paris-Saclay and Inserm. The results may lead to the development of new drugs, have been patented and were recently published in the journal Nature Communications.
Antibiotic resistance is a major challenge for our healthcare systems. According to the WHO, 5 million people worldwide die every year as a result of antibiotic resistance. By 2050, this could become the most common cause of death worldwide.
Although antibiotics have significantly reduced mortality associated with infectious diseases, their sometimes excessive and abusive use has led to the development of bacterial resistance. Most antibiotics have a broad spectrum of action and target several important metabolic pathways of bacteria. They do not have a specific effect and therefore attack all bacteria - even the beneficial ones - in the host. There is an urgent need to identify and characterise new bacterial targets for drugs. Researchers from the participating institutions are working on the development of innovative anti-infectives to specifically combat pathogenic bacteria.
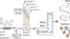
In their study, the researchers identified the so-called Mfd protein (Mutation Frequency Decline). A virulence factor that is produced by all bacteria and is essential for them to resist the host's immune system. This protein has the additional function of promoting spontaneous and random mutations that increase the bacteria's ability to develop resistance.
Mitigating invading bacteria and protecting the microbiota
The interdisciplinary research team from INRAE, CNRS, University of Paris-Saclay, Inserm and the CMFI Cluster of Excellence at the University of Tübingen wanted to find and develop an active substance that could block the Mfd protein and thus ‘disarm’ the pathogenic bacteria. The lead author, Nalini Rama Rao, worked on this project for two years as a visiting scientist in Lisa Maier's research group at the CMFI at the University of Tübingen.
From a library of 5 million molecules, the researchers identified a promising molecule called NM102, which is able to bind to the Mfd protein and prevent its activation.
In vitro and subsequent in vivo studies in insect and mouse models revealed the three main effects of the molecule:
1. it does not kill bacteria in the absence of toxic compounds produced by the immune system.
2. it reduces the amount of pathogenic bacteria in the infected organs without damaging the host microbiota
3. it is able to block the function of Mfd as a mutation factor, thereby reducing the ability of bacteria to develop resistance to antimicrobial agents.
The molecule is therefore able to ‘neutralise’ pathogenic bacteria and at the same time protect the host's other bacteria.
It is particularly promising that this molecule is also effective against bacterial strains that are resistant to current treatments and originate from hospitalised patients.
“This work represents an exciting shift in how we approach infectious disease treatment. Instead of killing bacteria indiscriminately, we’re targeting their ability to evade the immune system – without disrupting the host microbiome. This precision not only reduces the risk of resistance but also preserves the beneficial microbes essential to human health. It’s a promising step toward smarter, more sustainable anti-infective strategies,” says Lisa Maier, CMFI Board Member and co-author of the study.
From molecule to drug
The team has encapsulated the molecule in biodegradable nanoparticles to facilitate its administration. They are currently working with the French Alternative Energies and Atomic Energy Agency (CEA) on the chemical optimisation of analogue molecules. Two patents have been filed for the identification of the bacterial target and for the identification of the molecule itself.
Original publication
Tran S. L., Lebreuilly L., Cormontagne D. et al. (2025). An anti-virulence drug targeting the evolvability protein Mfd protects against infections with antimicrobial resistant ESKAPE pathogens. Nature Communications, DOI: https://doi.org/10.1038/s41467-025-58282-8.
(Source: INRAE press release, 29.04.2025.)
Prof. Dr. Lisa Maier
University of Tübingen
Interfaculty Institute for Microbiology and Infection Medicine
Cluster of Excellence CMFI
Microbiome-host interactions
E-Mail: l.maier @
@ uni-tuebingen.
uni-tuebingen. de
de
Website
Leon Kokkoliadis
Public Relations Management
Tel: +49 7071 29-74707 / +49 152 346 79 269
E-Mail: leon.kokkoliadis@uni-tuebingen.de

Deutschlandfunk Nova



The Conversation



The Conversation





Springer Nature



Springer Nature

